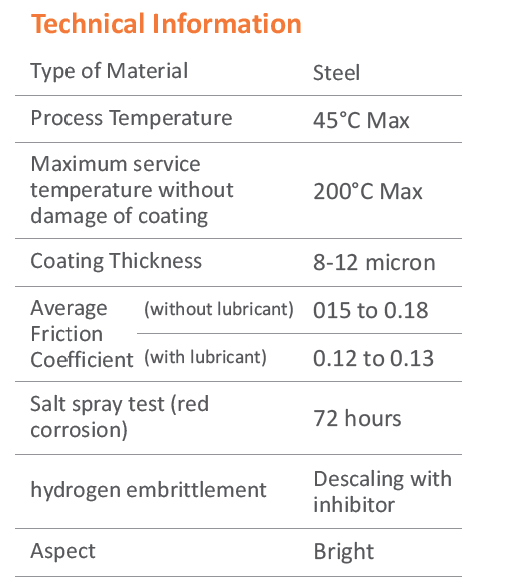
Zinc Electroplating
Zinc Electroplating provides corrosion resistance by acting as a barrier and sacrificial coating. Because zinc is more reactive that steel, the zinc coating corrodes first, protecting the steel substrate. The rate of corrosion of zinc is atleast 10 times slower than that of steel, thus a thin coating of zinc can protect steel for long time.
Electroplating process
Electroplating process starts with carefully cleaning the fastener surface in alkaline detergent type solutions. It is then treated with acid, in order to remove any rust or surface scales. Thorough cleanliness is essential as the molecular layers of oil or rust can prevent adhension of the coating to the metal surface.
Electroplating is done by the process of electro deposition. The fasteners are dipped in a chemical bath containing dissolved zinc. When direct current is applied, the zinc metal at the anode begins to dissolve, and the free metalions reach the cathode to form a thin layer of coating on the fastener. The thickness of zinc plating depends on the time spent in the plating bath, the amount of electric current, and the chemical composition of bath.
Advantages
Electroplating results in smooth, shiny & drip-free surface - preferable for aesthetic reasons. Because it's thin - It doesn't interfere with fastener threads. Also has a significantly lower cost.
Hydrogen Embrittlement
During acid cleaning and in the electroplating process, atomic hydrogen produced can diffuse into the steel and embrittle the structure of fastener. The electroplated coating traps the hydrogen inside the fasteners, which can migrate to areas of high stress and cause small microcracks & ultimately lead to brittle failure, unless they are baked soon after plating to drive the hydrogen out. High strength fasteners are particularly prone to hydrogen embrittlement because greater the strength (or hardness) of the alloy fasteners, the greater the susceptibility to hydrogen damage failure.
At Unbrako the post-plating de-embrittlement baking is carried out on Fully Automatic Electroplating line with synchronized Auto loading system & hydrogen De-Embrittlement furnace & Passivation line. Thus no time is wasted in - between the processes, allowing for highest quality plating.
Passivation
Passivation is the use of light coat of material such as metal oxide to create a shell against environmental factors such as air or water i.e. corrosion. Passivation is useful in strengthening and preserving the appear of metal.
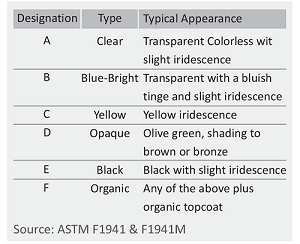
Chromate Conversion Coatings
For use in moderate or severe environments, fasteners may be chromate-conversion coated for additional corrosion protection. Clear, blue - bright, designation A or B trivalent chromates are normally used for Grade 5 or Class 8.8 fasteners. Grade 8 or Class 10.9 fasteners are usually supplied with yellow chromate C3+ is hex avalent free.
Unbrako is ISO 9001 & TS 16949 certified. At Unbrako, we take a number of precautions to make sure over fasteners meet or exceed governing specifications. Further, full traceability to original manufacturing lot number is available.











